
Definition of Hardness
To understand hardness, we should first ask why the concept was created. Hardness emerged around the late 19th to early 20th century. During the Industrial Revolution, boilers and steam engines suffered explosions as scale accumulated, and everyday problems arose because soap would not dissolve properly in water. The cause was traced to the water itself, and experiments with soap were used to characterize its properties. The harder the water, the more poorly soap dissolved and the more soap was required. Hence, water that was “difficult to work with” came to be called “hard water.” The ions chiefly responsible were calcium (Ca²⁺) and magnesium (Mg²⁺).
Today, hardness refers to how “hard” it is for soap to dissolve and is determined by the concentrations of calcium and magnesium ions. These ions form insoluble soap scum by reacting with the fatty acids in soap. Because calcium and magnesium do not contribute equally to this effect, their contributions are adjusted separately. To normalize the differing effects, researchers adopted calcium carbonate (CaCO₃) as a common reference and derived a conversion formula.
While conventions vary by country, hardness is commonly reported in mg/L as CaCO₃ (or ppm as CaCO₃). The phrase “as CaCO₃” indicates normalization to the calcium-carbonate standard. The conversion is as follows: multiply calcium concentration by ≈2.5 and magnesium concentration by ≈4.1 to obtain hardness expressed as CaCO₃.
For example, if [Ca²⁺] = 50 ppm and [Mg²⁺] = 30 ppm, then hardness ≈ 2.5 × 50 + 4.1 × 30 ≈ 250 ppm as CaCO₃.
Hardness = 2.5 × [calcium ion] + 4.1 × [magnesium ion]
Temporary Hardness and Permanent Hardness
Hardness is classified as temporary or permanent. As the name suggests, temporary hardness is transient and can be removed easily—often simply by boiling the water. Permanent hardness, by contrast, does not diminish readily upon boiling. The sum of the two is the total hardness.
The difference between temporary and permanent hardness lies in the “conditions.” In both cases, the causative ions are the same—calcium and magnesium. Depending on the environment in which these ions exist, hardness can manifest as temporary or permanent.
Temporary hardness
Temporary hardness diminishes upon boiling because calcium and magnesium react with bicarbonate (HCO₃⁻) in water. Insoluble precipitates such as calcium carbonate (CaCO₃) and magnesium carbonate (MgCO₃) are formed; we commonly refer to this deposit as “scale.” After prolonged use of an electric kettle, such scale is visible on the bottom—an outcome of this reaction. As dissolved calcium and magnesium are removed from solution, hardness decreases. Because bicarbonate is consumed, alkalinity also declines. In short, temporary hardness refers to the condition in which calcium and magnesium coexist with bicarbonate. It is therefore also called “carbonate hardness.”
Ca²⁺ + 2 HCO₃⁻ —(heat)→ CaCO₃↓ + CO₂↑ + H₂O
The equation above shows calcium ions reacting with bicarbonate to form calcium carbonate; the product precipitates while carbon dioxide and water are generated.
Permanent hardness
If boiling does not reduce hardness, it indicates the absence of bicarbonate in the water. In such cases, chloride (Cl⁻) or sulfate (SO₄²⁻) are typically present instead. These anions do not react with calcium or magnesium under heating, so the hardness-causing ions remain in solution and hardness is not reduced—this is permanent hardness. Because carbonate does not participate, permanent hardness is also called “non-carbonate hardness.”
Total hardness (General Hardness, GH)
Total hardness is the sum of temporary and permanent hardness. In practice, only one type may be present, or both may coexist. For example, if sufficient bicarbonate is present to convert all calcium ions into calcium carbonate upon prolonged boiling, no calcium ions remain in solution and hardness becomes zero; in this case, temporary hardness alone exists and equals total hardness. Conversely, if no bicarbonate is present, boiling will not form calcium carbonate; only permanent hardness exists and equals total hardness. Finally, if calcium is abundant but bicarbonate is relatively scarce, extended boiling will consume the available bicarbonate and remove some fraction of the hardness as calcium carbonate, yet residual calcium will remain that cannot be removed by boiling. In that case, temporary and permanent hardness coexist, and their sum is the total hardness.
Relationship between hardness and alkalinity
An interesting relationship exists among total hardness, temporary hardness, permanent hardness, and alkalinity. Temporary and permanent hardness are not absolute; they depend on “conditions,” and those conditions are set by alkalinity. Depending on the level of alkalinity, the proportions of temporary and permanent hardness will change. Consider two scenarios:
• If total hardness exceeds alkalinity: calcium and magnesium are relatively abundant, while bicarbonate is comparatively scarce. If total hardness is 100 ppm and alkalinity is 70 ppm, then up to 70 ppm of the hardness-causing ions can react with bicarbonate to form calcium carbonate and be removed. Temporary hardness is therefore 70 ppm, and the remaining 30 ppm is permanent hardness.
• If total hardness is less than alkalinity: bicarbonate is relatively abundant compared with calcium and magnesium. If total hardness is 70 ppm and alkalinity is 100 ppm, then all hardness-causing ions will be removed by reaction with bicarbonate. In this case, temporary hardness equals total hardness (70 ppm), and permanent hardness is 0 ppm.
Summary of relationships among total hardness, temporary hardness, permanent hardness, and alkalinity
Case 1: Total hardness > Alkalinity
Temporary hardness = Alkalinity
Permanent hardness = Total hardness − Alkalinity
Case 2: Total hardness < Alkalinity
Total hardness = Alkalinity
Permanent hardness = 0
Rate of decrease of temporary hardness and alkalinity under heating
The rate at which temporary hardness and alkalinity decline depends on how effectively conditions favor the reaction between calcium ions and bicarbonate. Heating promotes calcium carbonate formation. Initial ion concentrations, pH, and other variables also substantially influence how quickly temporary hardness and alkalinity decrease.
Across a range of studies, temporary hardness and alkalinity decrease most rapidly during the first ~10 minutes after boiling begins. The rate then tapers off, approaching a near-equilibrium after ~20 minutes. In households or cafés, water for brewing is typically heated to 100 °C for around 10 minutes. After ~15 minutes at a rolling boil, temporary hardness often decreases by roughly 60% to as much as 80%. If one aims to lower temporary hardness and alkalinity reliably, prolonged boiling is advantageous; conversely, if one wishes to preserve them, heating should stop just before boiling.
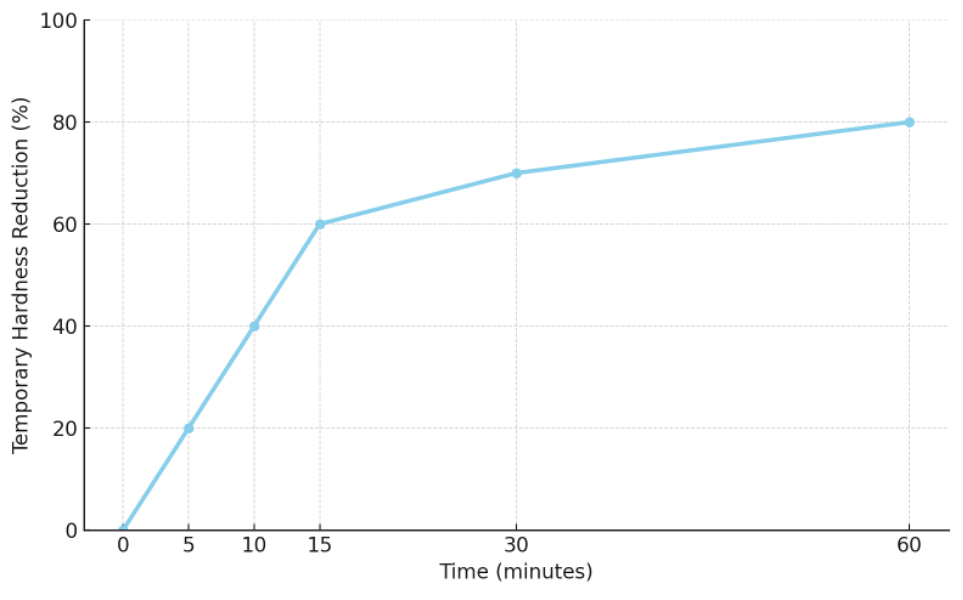
(Rate of decrease in temporary hardness during heating at 100 °C)
Since the 2020s, most specialty-coffee professionals no longer bring brewing water to a rolling boil for hand-brewing. Electric kettles that heat and hold at a set temperature are widely used, and the data above do not apply in those cases. Fifteen minutes at 100 °C (a vigorous boil) is very different from 15 minutes at 90–95 °C. If temporary hardness decreases by about 60% in 15 minutes at 100 °C, the decrease is only ~40% at 95 °C and ~30% at 90 °C. The key is carbon dioxide: compared with 90–95 °C, CO₂ is released far more vigorously at a rolling boil. As CO₂ degasses, the reaction that converts calcium and bicarbonate into calcium carbonate is driven forward to replenish the lost CO₂. Additionally, unlike many solids, calcium carbonate becomes less soluble as temperature increases, so material dissolved at 90 °C may precipitate at 100 °C. Nevertheless, CO₂ degassing is the dominant driver of temporary-hardness reduction.
Ca²⁺ + 2 HCO₃⁻ —(heat)→ CaCO₃↓ + CO₂↑ + H₂O
If a café uses water from a hot-water dispenser for hand-brew, this topic becomes especially relevant, because such heaters maintain water at elevated temperatures for many hours. In practice, water stored in a heater may experience some reduction in hardness and alkalinity, though not necessarily dramatic. Generally, hot-water tanks do not maintain a rolling boil; without vigorous boiling, CO₂ degassing is limited, pH does not rise, and bicarbonate does not readily convert to carbonate (CO₃²⁻). Calcium carbonate formation is favored at higher pH and higher carbonate concentrations, conditions that are not strongly met in typical hot-water tanks. That said, temperature matters: a tank at 85 °C behaves very differently from one at 65 °C. Many scaling studies cite ~60 °C as a threshold for scale formation and consider 65–70 °C and above a risk zone. Roughly speaking, 65 °C is just above the onset of scale formation, and only modest reductions in temporary hardness occur. By contrast, in a simple model based on CO₂ degassing, 85 °C water held for ~26–32 hours could see >90% of temporary hardness removed, whereas at 65 °C over the same duration the reduction may be only ~20–30%.
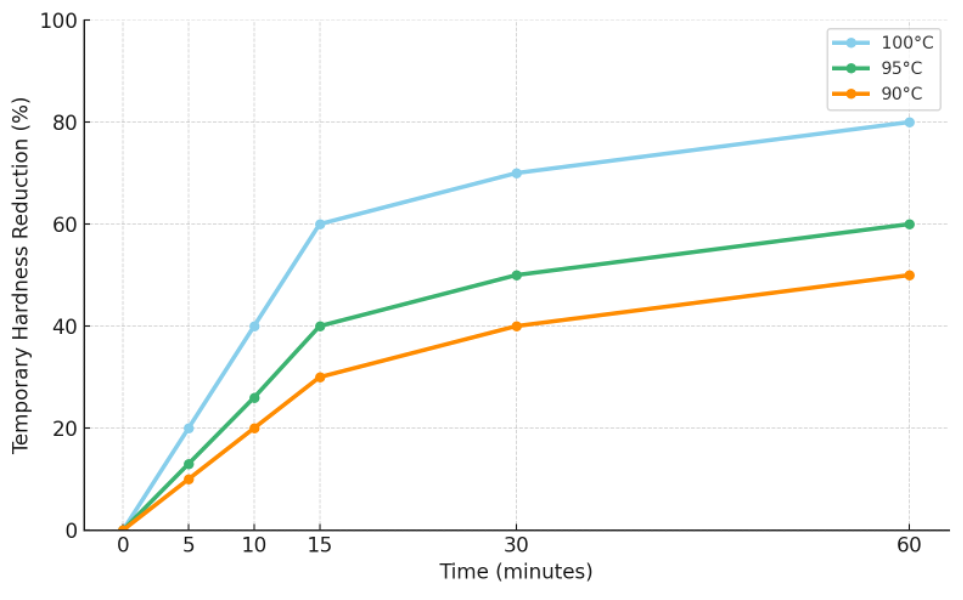
(Decrease in temporary hardness versus temperature and time)
Because storage conditions vary widely, it is generally advisable not to use hot-water-tank water for hand-brew. If a tank is set between 85–95 °C, temporary hardness and alkalinity may fall substantially. Since cafés seldom draw a fixed daily volume, the chemistry of stored water will vary day to day. If yesterday’s service was slow, today’s tank water may show lower hardness; if today is busy, tomorrow’s may be higher. Regardless of whether the water tastes “better” or “worse,” using water of shifting composition is not ideal for a shop serving beverages. A prudent approach is to reserve tank water for Americanos and tea service.
The most reliable method for preparing hand-brew water is to draw fresh water as needed and heat it only to the target temperature—avoid a rolling boil. If you plan to brew at 93 °C, heat only to 93 °C; for 85 °C, heat only to 85 °C. Minimizing heating time limits pH drift and CO₂ loss and therefore avoids altering temporary hardness. If brewing begins immediately upon reaching the target temperature, reductions in temporary hardness and alkalinity are typically under 5% and can be considered negligible. However, holding water at temperature for extended periods will change its chemistry and should be avoided.
Hard and Soft Water
Water with high hardness is called hard water; water with low hardness is called soft water. There is no single academic or scientific threshold that universally separates the two. Because the concept of hardness originated from practical concerns—scale formation and soap performance—classification varies by country and institution. In Korea, regulatory limits emphasize potability: tap water hardness must be ≤300 ppm as CaCO₃, and bottled water ≤500 ppm as CaCO₃.
Classification by hardness (unit: ppm as CaCO₃)
- Korea: Soft 0–90; Hard ≥200
- Japan: Soft 0–60; Slightly hard 61–120; Hard 121–180; Very hard >180
- USA: Soft 0–60; Slightly hard 61–120; Hard 121–180; Very hard >180
- UK: Soft 0–50; Slightly hard 51–100; Hard 101–200; Very hard >200
- Germany: Soft 0–150; Moderately hard 150–250; Hard >250
- France: Soft 0–80; Moderately hard 80–150; Hard 150–300; Very hard 300–400; Extremely hard >400
- Italy: Very soft 0–40; Soft 41–80; Moderately hard 81–120; Hard 121–180; Very hard 181–300; Extremely hard >300
The Relationship Between Hardness and Coffee Flavor
Hardness affects not only the taste of the water itself but also the final flavor of coffee. High-hardness water can feel sharp and dense; low-hardness water can seem dilute or light. However, as with TDS, hardness lacks crucial detail: its two principal contributors—calcium and magnesium—play different roles. Thus, three waters with the same total hardness of 100 ppm (one composed entirely of magnesium hardness, one entirely of calcium hardness, and one a 50:50 split) will produce coffees with distinctly different flavor profiles. For practical purposes, what helps is knowing the respective levels of calcium and magnesium rather than only their combined hardness. Because hardness aggregates these ions indiscriminately, it is of limited direct use for predicting extraction and flavor on its own.
There has been a hypothesis—still widely believed among baristas—that divalent cations increase extraction of coffee compounds. Yet if the claim is simply that “more coffee compounds” are extracted, academic support remains limited, and in the CoffeeLink Experiment, it was difficult to detect meaningful concentration differences among samples at 50, 100, 150, and 200 ppm hardness. Sensory results, however, told a different story: 97% of panelists reported perceivable sensory differences (aroma, acidity, flavor, texture) as hardness varied, with 3% finding discrimination difficult only in aroma. Notably, 100% of panelists perceived differences between 50 ppm and 400 ppm samples. Between samples composed of 100% calcium hardness and 100% magnesium hardness at the same total hardness of 200 ppm, 67% reported similar overall flavor intensity, but 98% reported clear differences in flavor character; all panelists reported textural/body differences. These findings support the view that hardness alone cannot determine extraction outcomes.
In terms of preference within the CoffeeLink Experiment (≈100 professional tasters), the most favored ratio of hardness contributors was calcium:magnesium at 7:3, followed by 6:4.
Even though hardness originated from considerations of soap performance rather than beverage extraction, it remains practically valuable in coffee because it is essential to managing expensive equipment. The internal plumbing of coffee machines and water heaters is narrow and vulnerable; even small amounts of scale can cause failures, and repairs are costly. The near-boiling environments inside espresso machines dramatically increase scaling risk. Café owners should monitor water quality regularly for changes in hardness. The risk of scale rises with higher temporary hardness—that is, when calcium and bicarbonate coexist at elevated levels. Water quality management is therefore not only a taste-quality issue but a business and maintenance concern.
Although hardness does not disclose exact mineral contents, it still provides more specific information than TDS, which lumps all dissolved solids together. Hardness offers an indirect read on the two key divalent cations. Just as extremely low or high TDS warrants caution, so too do extreme hardness values. Waters below ~30 ppm or above ~200 ppm are not recommended. The Specialty Coffee Association (SCA) recommends total hardness in the range of approximately 50–175 ppm as CaCO₃.